The systematic name for the compound Mn₂ (SO₄)₃ is manganese (III) sulfate. In this compound, manganese has a +3 oxidation state as indicated by the Roman numeral III. Each sulfate (SO₄²-) ion has a -2 charge.
Red book 2005 by Ricardo Ubillus Ruiz – Issuu
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Source Image: pubs.acs.org
Download Image
Example 6. Write the systematic name (and the common name if applicable) for each ionic compound. LiCl; MgSO 4 (NH 4) 3 PO 4; Cu 2 O; Given: empirical formula Asked for: name Strategy: A If only one charge is possible for the cation, give its name, consulting Table 4.3.1 or Table 4.4.1 if necessary. If the cation can have more than one charge (Table 4.4.1), specify the charge using roman numerals.

Source Image: numerade.com
Download Image
Spell out the name of the compound in the image below. | Homework.Study.com
Name: Manganese(III) Sulfate. Alias: Manganic Sulfate. Formula: Mn2(SO4)3. Molar Mass: 398.0639. Mn2(SO4)3 Molar Mass Converter. Weight: Mole: Example Reactions: • 2 Mn + 3 CuSO4 = Mn2(SO4)3 + 3 Cu • 8 HI + 2 KMnO4 + 4 H2SO4 = 4 I2 … Compound Name Formula Search
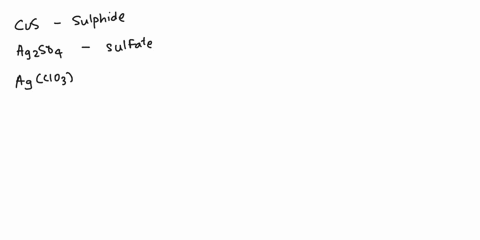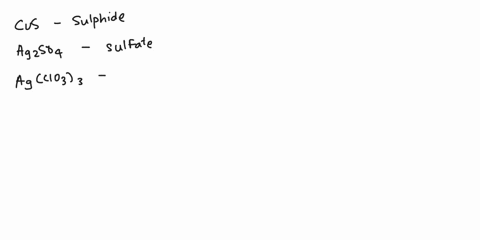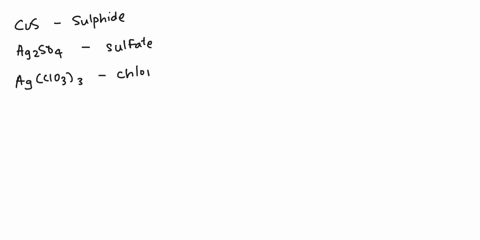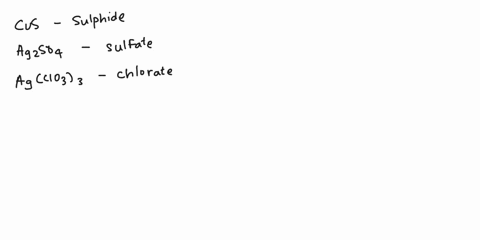
Source Image: numerade.com
Download Image
Give The Systematic Name For The Compound Mn2 So4 3
Name: Manganese(III) Sulfate. Alias: Manganic Sulfate. Formula: Mn2(SO4)3. Molar Mass: 398.0639. Mn2(SO4)3 Molar Mass Converter. Weight: Mole: Example Reactions: • 2 Mn + 3 CuSO4 = Mn2(SO4)3 + 3 Cu • 8 HI + 2 KMnO4 + 4 H2SO4 = 4 I2 … Compound Name Formula Search
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Mn2 (SO4)3: Molar Mass (g/mol) Mn (Manganese) 2 × 54.938045 = 109.87609. S (Sulphur/Sulfur) 3 × 32.065 = 96.195. O (Oxygen)
SOLVED: Give systematic names to the chemical formulas given below? Formula Name Mn2(SO4)3 Ca3(PO3)2 CuBr2 Co4(SiO4)3 S2Br3 As2Cl3 N2O3 CrBr3·6H2O
Sep 15, 2023Final answer: The systematic name for the compound Mn₂ (SO₄ )₃ is manganese (III) sulfate. Explanation: The systematic name for the compound Mn₂ (SO₄ )₃ is manganese (III) sulfate. In this compound, there are two manganese (Mn) atoms and three sulfate (SO₄) ions.
In the reaction MNO4- + SO3²- + H+ → SO4² – +MN² + H2O, which is oxidised and which is reduced? – Quora
Source Image: quora.com
Download Image
Solved Which of the following is the correct formula for | Chegg.com
Sep 15, 2023Final answer: The systematic name for the compound Mn₂ (SO₄ )₃ is manganese (III) sulfate. Explanation: The systematic name for the compound Mn₂ (SO₄ )₃ is manganese (III) sulfate. In this compound, there are two manganese (Mn) atoms and three sulfate (SO₄) ions.
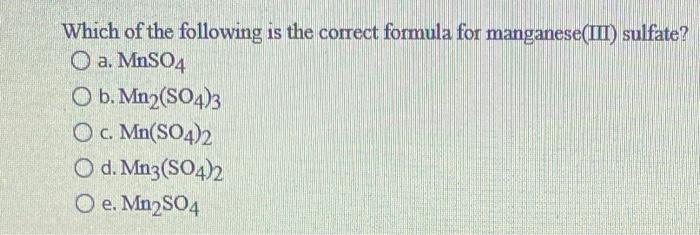
Source Image: chegg.com
Download Image
Red book 2005 by Ricardo Ubillus Ruiz – Issuu
The systematic name for the compound Mn₂ (SO₄)₃ is manganese (III) sulfate. In this compound, manganese has a +3 oxidation state as indicated by the Roman numeral III. Each sulfate (SO₄²-) ion has a -2 charge.

Source Image: issuu.com
Download Image
Spell out the name of the compound in the image below. | Homework.Study.com
Example 6. Write the systematic name (and the common name if applicable) for each ionic compound. LiCl; MgSO 4 (NH 4) 3 PO 4; Cu 2 O; Given: empirical formula Asked for: name Strategy: A If only one charge is possible for the cation, give its name, consulting Table 4.3.1 or Table 4.4.1 if necessary. If the cation can have more than one charge (Table 4.4.1), specify the charge using roman numerals.

Source Image: homework.study.com
Download Image
Manganese(II) sulfate | MnO4S | ChemSpider
Solution for Give the systematic name for the compound Mn2 (SO4)3.
Source Image: chemspider.com
Download Image
Solved Name Tues @ 11 Name Formula or Mn2(SO3)3 ammonium | Chegg.com
Name: Manganese(III) Sulfate. Alias: Manganic Sulfate. Formula: Mn2(SO4)3. Molar Mass: 398.0639. Mn2(SO4)3 Molar Mass Converter. Weight: Mole: Example Reactions: • 2 Mn + 3 CuSO4 = Mn2(SO4)3 + 3 Cu • 8 HI + 2 KMnO4 + 4 H2SO4 = 4 I2 … Compound Name Formula Search
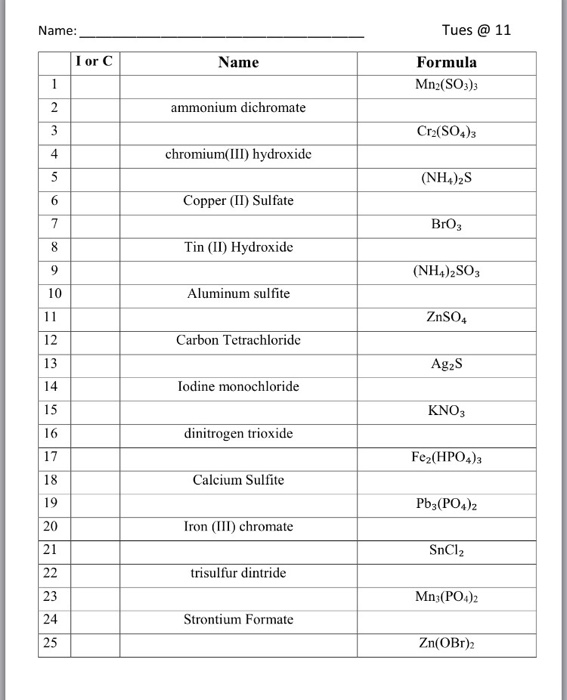
Source Image: chegg.com
Download Image
Solved I or C Formula Mn2(SO3)3 Name ammonium chromate CrSO3 | Chegg.com
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Mn2 (SO4)3: Molar Mass (g/mol) Mn (Manganese) 2 × 54.938045 = 109.87609. S (Sulphur/Sulfur) 3 × 32.065 = 96.195. O (Oxygen)
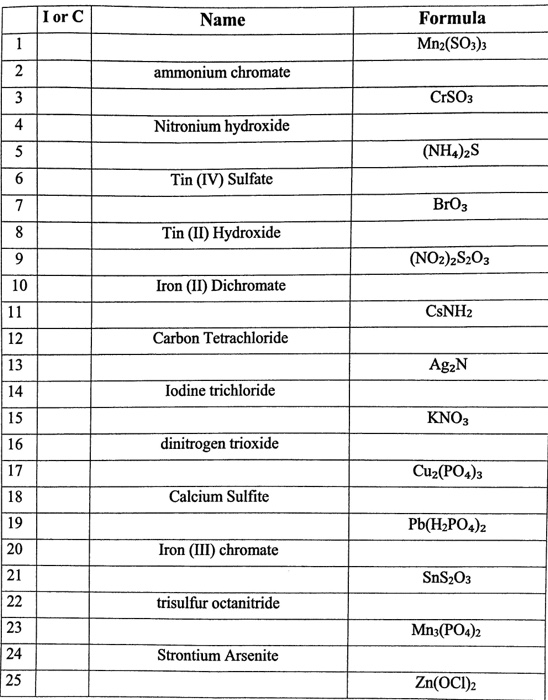
Source Image: chegg.com
Download Image
Solved Which of the following is the correct formula for | Chegg.com
Solved I or C Formula Mn2(SO3)3 Name ammonium chromate CrSO3 | Chegg.com
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Spell out the name of the compound in the image below. | Homework.Study.com Solved Name Tues @ 11 Name Formula or Mn2(SO3)3 ammonium | Chegg.com
Solution for Give the systematic name for the compound Mn2 (SO4)3.