The decomposition of ozone in the atmosphere is proposed to occur via the following mechanism:Step 1: Cl(g) + O3(g) → ClO(g) + O2(g)Step 2: ClO(g) + O3(g) → Cl(g) + 2 O2(g)Identify the intermediate. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list.
Solved A proposed mechanism for the decomposition of ozone | Chegg.com
Reaction Mechanism practice set. The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism. Step 1: O31g2ΔO21g2 + O1g2 (fast) (b) Derive the rate law that is consistent with this mechanism. (Hint: The product appears in the rate law.)
Source Image: chegg.com
Download Image
The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs. The decomposition of ozone, for example, appears to follow a mechanism with two steps: O3(g) O2(g) + O O +O3(g) 2O2(g) O 3 ( g) O 2 ( g) + O O + O 3 ( g) 2 O 2 ( g)

Source Image: youtube.com
Download Image
The effect of temperature and peroxides content on cross-linking and properties of EPDM-based rubber matrix – Ján Kruželák, Andrea Kvasničáková, Klaudia Hložeková, Michaela Džuganová, Ivan Hudec, 2022 VIDEO ANSWER: The first step of the ozone decomposition reaction is slower than the second step, which is an elementary reaction. This is our slow step, and this is our fast step, and we know that the rate determining step is the one that affects the
![Kannada] The decomposition of ozone proceeds as : O(3) rarr O(2) +](https://static.doubtnut.com/ss/web-overlay-thumb/9307961.webp)
Source Image: doubtnut.com
Download Image
Suppose The Decomposition Of Ozone Proceeds By The Following Mechanism
VIDEO ANSWER: The first step of the ozone decomposition reaction is slower than the second step, which is an elementary reaction. This is our slow step, and this is our fast step, and we know that the rate determining step is the one that affects the VIDEO ANSWER: The first step of the ozone decomposition reaction is slower than the second step, so we have a 2 step mechanism where each step is an elementary reaction. The rate law affects the rate at which the whole reaction happens and that is
Kannada] The decomposition of ozone proceeds as : O(3) rarr O(2) +
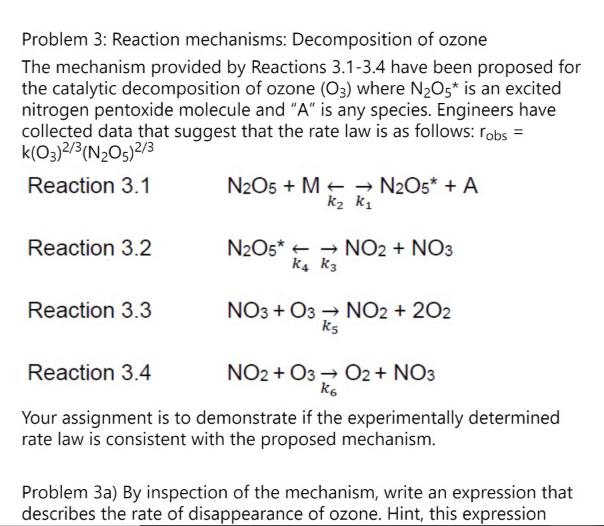
Oct 27, 2022The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. A chemical reaction often occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps: \[\ceO3(g) \ceO2(g)+\ceO\\ Problem 3: Reaction mechanisms: Decomposition of | Chegg.com
Source Image: chegg.com
Download Image
1 Mechanisms of ozone decomposition in water | Download Table Oct 27, 2022The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. A chemical reaction often occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps: \[\ceO3(g) \ceO2(g)+\ceO\\

Source Image: researchgate.net
Download Image
Solved A proposed mechanism for the decomposition of ozone | Chegg.com The decomposition of ozone in the atmosphere is proposed to occur via the following mechanism:Step 1: Cl(g) + O3(g) → ClO(g) + O2(g)Step 2: ClO(g) + O3(g) → Cl(g) + 2 O2(g)Identify the intermediate. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list.

Source Image: chegg.com
Download Image
The effect of temperature and peroxides content on cross-linking and properties of EPDM-based rubber matrix – Ján Kruželák, Andrea Kvasničáková, Klaudia Hložeková, Michaela Džuganová, Ivan Hudec, 2022 The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs. The decomposition of ozone, for example, appears to follow a mechanism with two steps: O3(g) O2(g) + O O +O3(g) 2O2(g) O 3 ( g) O 2 ( g) + O O + O 3 ( g) 2 O 2 ( g)

Source Image: journals.sagepub.com
Download Image
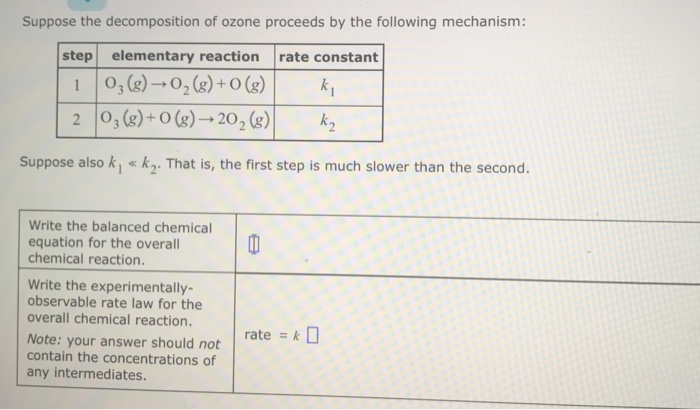
SOLVED: Suppose the decomposition of ozone proceeds by the following mechanism: step elementary reaction rate constant 03(g) Yt 02(g) + O(g) k1 2 03(g) O(g) 202(9) k2 Suppose also k1

Source Image: numerade.com
Download Image
Suggest a mechanism for the decomposition of ozone, O_3 into O_2. | 12 | CHEMICAL KINETICS | CHE… – YouTube VIDEO ANSWER: The first step of the ozone decomposition reaction is slower than the second step, which is an elementary reaction. This is our slow step, and this is our fast step, and we know that the rate determining step is the one that affects the

Source Image: m.youtube.com
Download Image
Chemical Kinetics-I: Part – I: Subjective Questions | PDF | Activation Energy | Chemical Kinetics VIDEO ANSWER: The first step of the ozone decomposition reaction is slower than the second step, so we have a 2 step mechanism where each step is an elementary reaction. The rate law affects the rate at which the whole reaction happens and that is

Source Image: scribd.com
Download Image
1 Mechanisms of ozone decomposition in water | Download Table
Chemical Kinetics-I: Part – I: Subjective Questions | PDF | Activation Energy | Chemical Kinetics Reaction Mechanism practice set. The gas-phase decomposition of ozone is thought to occur by the following two-step mechanism. Step 1: O31g2ΔO21g2 + O1g2 (fast) (b) Derive the rate law that is consistent with this mechanism. (Hint: The product appears in the rate law.)
The effect of temperature and peroxides content on cross-linking and properties of EPDM-based rubber matrix – Ján Kruželák, Andrea Kvasničáková, Klaudia Hložeková, Michaela Džuganová, Ivan Hudec, 2022 Suggest a mechanism for the decomposition of ozone, O_3 into O_2. | 12 | CHEMICAL KINETICS | CHE… – YouTube VIDEO ANSWER: They tell us that the first step is slower than the second because ozone is separated into two parts. Slow or rate determining step is what this is. The second step is taking 1 to become 2. The overall balanced reaction will be the sum